electron withdrawing groups|electron withdrawing effect : Tuguegarao An electron-withdrawing group (EWG) is a group or atom that has the ability to draw electron density toward itself and away from other adjacent atoms. This electron density transfer is often achieved by resonance or inductive effects. Security is of utmost importance when you’re trying to do online transactions, and this is where eChecks excel the most. Many online casinos in the US accept eChecks, providing players with safe, reliable, and low-fee payments.. If you prefer using eChecks for online gambling, check out our list of the best US eCheck casinos .
PH0 · inductive electron withdrawal
PH1 · electron withdrawing inductive effect
PH2 · electron withdrawing groups list
PH3 · electron withdrawing effect
PH4 · electron donating effect
PH5 · electron donating and withdrawing groups
PH6 · electron donating ability
PH7 · diels alder stereochemistry
PH8 · Iba pa
REVALIDATION (APIR) FOR GSIS PENSIONERS I. BACKGROUND/RATIONALE In 2018, the Government Service Insurance System (GSIS) implemented the . (APIR) Form (Annex A); b. Original UMID eCard, eCard Plus or in its absence at least two (2) valid government issued IDs; c. For survivorship pensioners, duly accomplished Self-declaration ofImages of Queen Miranda from Sofia the First. Disney Wiki. Explore. Main Page; Discuss; All Pages; Community; Interactive Maps; Recent Blog Posts; Films. Animated films. . King Roland and Miranda fall in love. Mother and daughter hug. the marriage of King Roland and Miranda. Miscellaneous []
electron withdrawing groups*******An electron-withdrawing group (EWG) is a group or atom that has the ability to draw electron density toward itself and away from other adjacent atoms. This electron density transfer is often achieved by resonance or inductive effects.Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. Examples of electron withdrawing .Because Lewis acid-base reactions involve electron donation and acceptance at particular sites, substituent groups which alter the electron density at a site through inductively .Electron donating groups are typically divided into three levels of activating ability (The "extreme" category can be seen as "strong".) Electron withdrawing groups are .
Deactivating groups withdraw the electrons away from the carbocation of the sigma complex causing destabilization and increasing the activation energy which slows down .In chemistry, the inductive effect in a molecule is a local change in the electron density due to electron-withdrawing or electron-donating groups elsewhere in the molecule, .Learn how to identify electron-withdrawing groups that enhance the electrophilicity of a carbonyl carbon in a substitution reaction. See an example question with answer .If the most common type of coupling partner contains electron-withdrawing groups (EWGs), the radical is considered nucleophilic, whereas if the most common partner .Radicals as Exceptional Electron-Withdrawing Groups: Nucleophilic Aromatic Substitution of Halophenols Via Homolysis-Enabled Electronic Activation. Nick Y. Shin. , Elaine Tsui. .electron withdrawing groups electron withdrawing effectElectron withdrawing group (EWG): An atom or group that draws electron density from neighboring atoms towards itself, usually by resonance or inductive effects. Trifluoro .
While heteroatom-centered radicals are understood to be highly electrophilic, their ability to serve as transient electron-withdrawing groups and facilitate polar reactions at distal sites has not been .
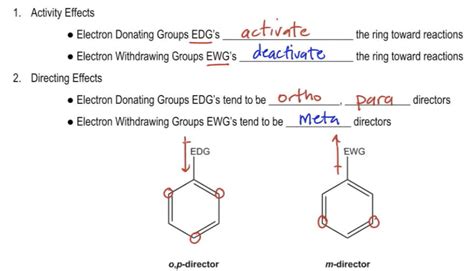
Just as electron-donating groups can stabilize a carbocation, electron-withdrawing groups act to destabilize carbocations. Carbonyl groups are electron-withdrawing by inductive effects, due to the polarity of the . 供电子基又叫electron donating group(EDG) 吸电子基又叫electron withdrawing group(EWG) 是一个通过改变电子密度影响化合物反应活性的重要因素。可以简单以两种方式来判断一个基团是EDG还是EWG。-----先讲第一种electron withdrawing effect In general, Diels-Alder reactions proceed fastest with electron-withdrawing groups on the dienophile (diene lover). Ethylene reacts slowly while propenal, ethyl propenoate, and other molecules shown below are highly reactive in a Diels-Alder reaction.. In much the same manner as electron-withdrawing substituents on a benzene ring, .electron withdrawing groupsElectron withdrawing group (EWG): An atom or group that draws electron density from neighboring atoms towards itself, usually by resonance or inductive effects. Trifluoro acetate ion is a weaker base than acetate ion because the trifluoromethyl group is attracting electron density away from the carboxylate . EWG, electron-withdrawing group. Full size image. Radical philicity and HAT chemistry. Radical philicity can influence the outcome of many types of organic reactions, .An electron withdrawing group or EWG draws electrons away from a reaction center. When this center is an electron rich carbanion or an alkoxide anion, the presence of the electron-withdrawing substituent has a stabilizing effect. Examples of electron withdrawing groups areElectron withdrawing group (EWG): An atom or group that draws electron density from neighboring atoms towards itself, usually by resonance or inductive effects. Trifluoro acetate ion is a weaker base than acetate ion because the trifluoromethyl group is attracting electron density away from the carboxylate .The nitro group (-NO 2), and the positively charged, tetra-substituted amino group (consider the structure once this trimethyl amino group is connected to the aryl ring) are both electron-withdrawing. As the trimethyl amino group will have an overall positive charge (and the nitro group is neutral overall), the trimethyl amino group is the .Inductive effect. In chemistry, the inductive effect in a molecule is a local change in the electron density due to electron-withdrawing or electron-donating groups elsewhere in the molecule, resulting in a permanent dipole in a bond. [1] It is present in a σ (sigma) bond, unlike the electromeric effect which is present in a π (pi) bond .Electron withdrawing group (EWG): An atom or group that draws electron density from neighboring atoms towards itself, usually by resonance or inductive effects. Trifluoro acetate ion is a weaker base .
The participation of the electron-withdrawing group in π-conjugation decreases the LUMO level and narrows the energy gap in the order of perfluoroalkyl, acyl, perfluoroacyl, nitro ≈ dicyanovinyl in both series. TD-DFT calculations allowed better understanding of electronic transitions.
Electron donating groups usually have lone pairs of electrons that can get mingled with the 6 pi electrons & take part in resonance. Electron withdrawing groups usually have an atom .

The ketone group is acting as an electron withdrawing group - it is 'pulling' electron density towards itself, through both inductive and resonance effects. Exercise 7.4.2. The position of the electron-withdrawing substituent relative to the phenol hydroxyl is very important in terms of its effect on acidity. Which of the two substituted . Shin, N. Y. et al. Radicals as exceptional electron-withdrawing groups: nucleophilic aromatic substitution of halophenols via homolysis-enabled electronic activation. J. Am. Chem.Effect of an electron withdrawing group in a benzyl cation. In this video, we see that an electron withdrawing group to a benzyl cation destabilises it. However adding it at ortho- and para- destabilises it more compared to meta- !! Created by Shahzad Karim. Are you able to explain this using molecular orbital theory?
The electron-withdrawing effect of the halogen atoms makes all of these acids stronger than butanoic acid. . so all these electron withdrawing groups if you will, are withdrawing electron density and that's stabilizing this conjugate base, that's spreading .
Learn Electron Withdrawing Groups with free step-by-step video explanations and practice problems by experienced tutors.
275 talking about this. We are the pioneer in the FRP market celebrating 65 years of service and excellence
electron withdrawing groups|electron withdrawing effect